Warning, the dangers of using equipment with stainless steel and sodium hydroxide
A detailed post on the dangers of using equipment that has stainless steel and sodium hydroxide. We describe why it's dangerous.
Jim Mitchell founder molecularhydrogenbubbles.com
12/2/20256 min read
Warning
The Hidden Dangers of Producing Hydrogen with Sodium Hydroxide and Stainless Steel.
Hydrogen gas has become a popular topic in health and wellness circles, with claims of anti-inflammatory and antioxidant benefits. Some enthusiasts have attempted to generate hydrogen gas at home using simple chemicals, often by reacting sodium hydroxide (NaOH, also known as lye) with metals like stainless steel. Many enthusiast with DIY hydrogen setups assume the gas produced is “pure” and safe to inhale,unfortunately, this is rarely the case.
A little backstory on myself. I have been building Brown’s gas, HHO generators as a hobbyist for over 15 years. I’ve made dozens of systems to provide supplemental HHO, Browns gas, oxyhydrogen to improve combustion and performance as a supplemental motor fuel. The picture below is the system that I built for human consumption. This is how I discovered molecular hydrogen for health, and at first I was using my own equipment, but as time went on, I began to realize the dangers of doing that. So when it comes to human consumption, I don’t believe this method of production is viable or healthy. This is why I’m writing this blog post about it. Some people are even marketing these browns gas generators to humans.
While it might sound straightforward, this DIY method is hazardous, and the potential risks are often underestimated. How does Sodium Hydroxide reacts with Stainless Steel you might be asking? Sodium hydroxide is a strong base capable of corroding metals. When combined with certain forms of stainless steel under heat or concentrated conditions, it can produce hydrogen gas. While hydrogen is being generated, the reaction is far from clean. Stainless steel contains iron, chromium, and sometimes nickel. The highly alkaline environment can leach metals from the steel, including hexavalent chromium (Cr⁶⁺), a known carcinogen.
Contaminants in the Gas
Sodium Hydroxide steam aerosols and hexavalent chromium can be detected in the gas stream. So it is very important that if you are using a gas generator that are using either one of these elements, sodium hydroxide or stainless steel, you will need to test your equipment to make sure that the filtration in the system is sufficient to remove these contaminants before you breathe the gas or bubble it into water making hydrogen rich water to drink.
When a browns gas electrolyzer is operating, it is creating heat enough heat to vaporize the water and NaOH [Lye] chemical into a steam aerosol. Hydrogen gas bubbling from a concentrated NaOH solution can carry tiny droplets of the caustic liquid. Inhaling these aerosols can cause severe irritation and chemical burns to the respiratory tract, throat, and lungs.
Metal Contamination
Stainless steel corrosion in strong NaOH solutions releases metals into the gas Hexavalent chromium, in particular, is highly toxic and carcinogenic. Even trace amounts inhaled over time can lead to lung damage, DNA mutations, and increased cancer risk.
How to test your equipment
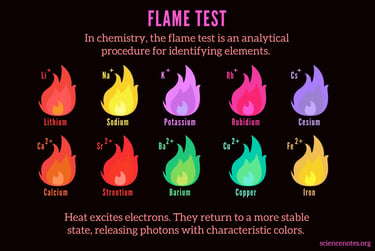
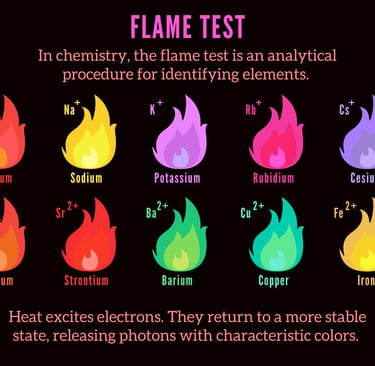
You can use a device called a bubbler which simply bubbles the gas output through distilled water. This will trap quite a bit of the sodium hydroxide and hexavalent chromium in the solution, but not all. We can test for sodium hydroxide with a simple flame color test. You can also test for the presence of hexavalent chromium by using a test strip in your bubbler water. If the strip changes color chromium six has made its way into your bubbler and on its way to you.
Using a torch tip, you can light the gas coming from the bubbler and check the color if it is yellow or orange it is contaminated with sodium hydroxide. When hydrogen is burning, the flame is invisible, or may have a blue hue to it. When invisible, you can feel the heat coming from the flame. The more pure the gas is, the more invisible it will burn. It is impurities in the gas that caused the gas to change color when it’s burning.
Other Metal Ions
Nickel and iron ions can also be present. While not as acutely toxic as Cr⁶⁺, chronic exposure can still lead to respiratory issues and allergic reactions.
Why is Sodium hydroxide and chromium six dangerous for Humans?
Chemical Burns due to direct exposure to NaOH, even in aerosol form, can severely damage tissues in the eyes, nose, mouth, and lungs.
Symptoms of chromium six exposure include irritation of the eyes, nose, and throat, leading to runny nose, sneezing, coughing, and nosebleeds. Prolonged exposure can cause more severe issues like skin and nasal ulcers, while inhalation may lead to asthma and an increased risk of lung cancer over time. Ingesting chromium six can cause stomach and intestinal irritation. Hexavalent chromium is particularly dangerous because it can cause oxidative damage to cells and DNA. Chronic exposure increases the risk of lung cancer, kidney damage, and liver damage.
I use this type of Brown’s gas electrolyzer myself for approximately four years and during that time I developed a tiny nasal ulcer that would not go away until I switched to a PEM electrolyzer that did not use sodium hydrogen or stainless steel. So I am operating with firsthand knowledge of these risks.
Explosive Risk
Browns gas, Oxyhydrogen, HHO, is a mixture of hydrogen and oxygen in the exact ratio needed for combustion, produced together during water electrolysis. Because the fuel (hydrogen) and the oxidizer (oxygen) are already blended, the gas ignites extremely easily and burns with a very fast flame speed, which can make it seem more “explosive.” Pure hydrogen by itself however, is not explosive until it mixes with oxygen from the air. On its own it is simply a fuel waiting for an oxidizer. The key difference is that Browns gas is premixed and ready to react, while hydrogen requires mixing with oxygen first meaning the apparent explosiveness comes from the presence of the oxidizer, not from hydrogen being inherently more powerful. Hydrogen is flammable generating it in an uncontrolled environment creates a serious risk of fire or explosion, especially if the gas concentration reaches 4–75% in air.
Safer Alternatives
Polymer exchange membrane (PEM) electrolyzers offer several advantages over standard Brown’s gas (HHO) oxyhydrogen electrolyzers, primarily due to their controlled, engineered separation of hydrogen and oxygen. Another advantage of a PEM system is that it has dual porting, one port for hydrogen and one port for oxygen. This gives you the option of hydrogen only or oxyhydrogen when blended together. You simply vent off the oxygen to the atmosphere and only consume the hydrogen.
In a PEM system, the solid membrane conducts protons while physically isolating the gases, improving both safety and product purity. This design allows for higher current densities and better electrical efficiency because the membrane minimizes ionic losses and enables more precise operating conditions. PEM units also avoid the caustic electrolytes common in older electrolyzer designs, reducing corrosion, simplifying maintenance, and enabling a more compact and durable system. Overall, PEM electrolyzers provide cleaner gas streams, higher performance, and enhanced reliability compared with Brown’s gas electrolyzers. These methods bypass the need for hazardous chemical reactions, ensuring the gas is free of NaOH and heavy metals like chromium six.
Titanium a better choice
Titanium offers several advantages over stainless steel when used in a gas electrolyzer.
It is far more resistant to corrosion in harsh electrochemical environments, especially where chlorine, oxygen, or acidic electrolytes are present, which greatly extends component lifetime. Titanium also forms a stable, protective oxide layer that prevents contamination of the electrolyte and maintains consistent performance over time. Its excellent strength to weight ratio allows for robust yet lightweight structures, and it maintains mechanical integrity at elevated temperatures common in electrolyzer operation. Although more expensive upfront, titanium’s durability and low maintenance requirements often make it the more cost effective choice for long term electrolyzer performance.
While the DIY approach of producing hydrogen gas using sodium hydroxide and stainless steel may seem appealing, it is unsafe. The gas is not “clean” it carries caustic sodium hydroxide, toxic metals like hexavalent chromium, and poses serious risks to respiratory and overall health. For anyone interested in the potential benefits of molecular hydrogen, using a highly engineered commercially manufactured products is the only safe route. As someone with many years of experience in these matters I do not recommend browns gas, HHO, or oxyhydrogen for human consumption.
In the next blog post, I will be explaining how to test even a manufactured product for purity so that you can have peace of mind that your product that you have purchased is safe and effective.
Written by
Jim Mitchell
Founder of molecularhydrogenbubbles.com.